Galvanic cells
A galvanic cell is an electrochemical cell that derives electrical energy from a spontaneous redox reaction. There are two beakers with electrolytic solutions in them, and one electrode in each. You need to be able to identify certain features in a galvanic cell:
- anode: the negative electrode
- cathode: the positive electrode
- oxidation half-cell: the beaker containing the anode
- reduction half-cell: the beaker containing the cathode
- salt bridge: a porous barrier that maintains electrical neutrality in the half-cells by allowing ions to flow through it
- electron flow: electrons flow from anode to cathode through a wire
- ion flow: anions flow through the salt bridge to the anode beaker; cations flow through the salt bridge to the cathode beaker
- voltmeter: a device that measures voltage
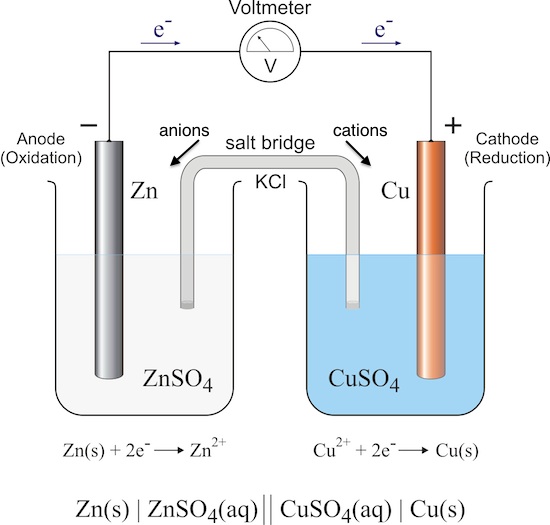
A galvanic cell can be represented succinctly with standard cell notation:
cathode | electrolyte || electrolyte | anode.
Sometimes, an inert electrode will be required because one of the half-reactions has no solids in it (the electrode has to be a solid). For example, a hydrogen–cobalt cell would be Pt(s) | H+(aq), H2(g) || Co2+(aq) | Co(s). At the anode, solid cobalt is flaking off and giving up electrons to become cobalt ions. In the other beaker, hydrogen ions are gaining electrons and becoming hydrogen gas. The electrons go through the wire and into the platinum electrode, which, unlike the cobalt electrode, does not participate in the reaction—it is inert. It’s sort of like sticking the wire into the solution, except that it doesn’t exactly work that way; it has to be an electrode made of platinum or carbon.
The key thing to remember with all of this is the associations:
- oxidation, increase oxidation number, loss of electrons, anode, negative
- reduction, decrease oxidation number, gain of electrons, cathode, positive